Capabilities and Sponsor Support: Latin America
Specialized in Rare Diseases, Immune Diseases, Cancer, Infectious Diseases
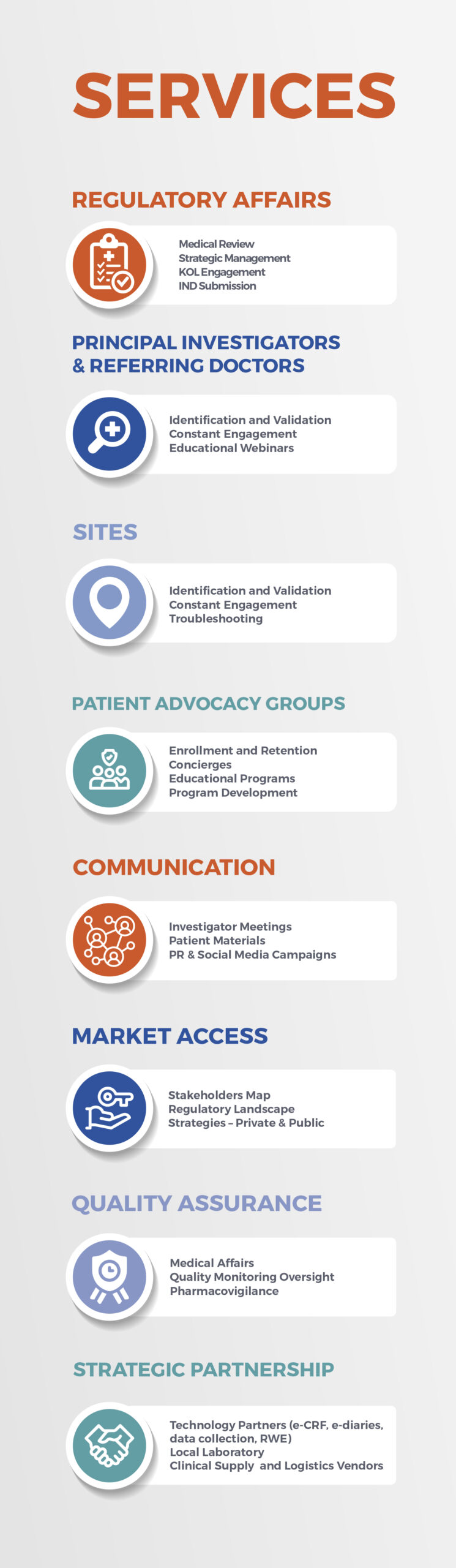
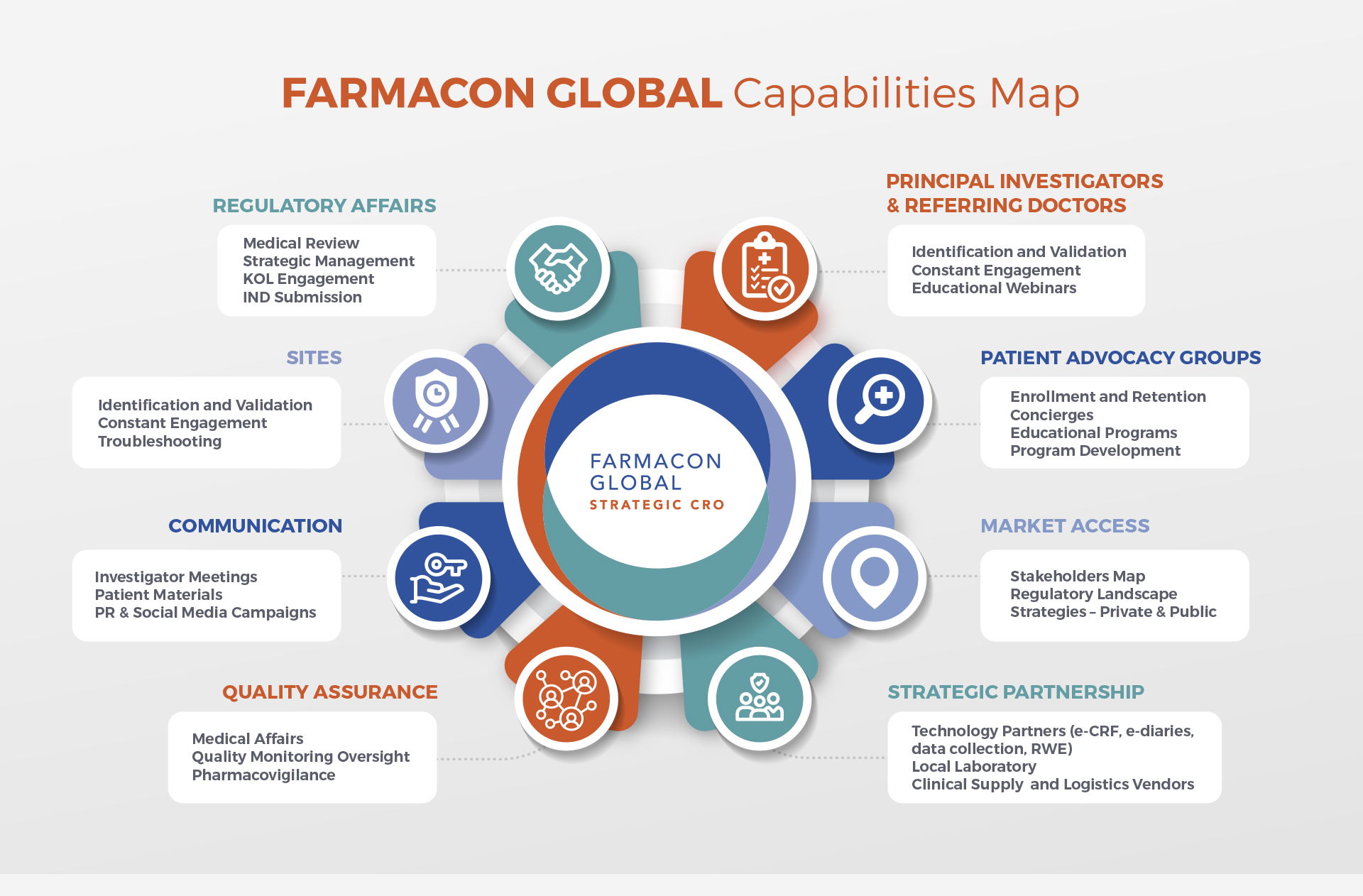
Clinical Trial Services for DIVERSITY Needs:
- Landscape reviews
- Regulatory support
- Protocol Development/Review
- Site identification & Engagement
- Diversity Enrollment Plans via Latin America and other Diverse countries
- PAG identification and Outreach via Doctor / PAG network – DIEM Alliance (Diseases in Emerging Markets)
- Enrollment plans &
Implementation - Medical Monitoring
- CRA Monitoring
Post Pivotal Clinical Trial:
- Pharmacovigilance
- Compassionate Use
- Advisory Boards for Biotechs/Pharmas
- Preceptorships for LatAm doctors to go to train with Specialists
- Auditing, FDA Audit Preparation
Best Practice Services
- Medical Consulting for any pre-clinical/clinical development needs
- Advisory boards
- Preceptorships
- RARE DIEM – conference to discuss Rare Diseases in Emerging Markets
- Health technology partners
Training
- Farmacon Academy – Training Platform
- High touch training
- PI/Sub-I/study coordinator
- CRO training
- CRA Monitor training
- Medical Monitor Training
- MSL training
- How to run Virtual Meetings