
“Through our partnership with Validcare we can offer the empir- ical results of more facile connections with our trial participants through e-CRF, e-diaries and more efficient RWE data being applied at a whole new level,” said Sara Tylosky, CEO, Farma- con Global. “Validcare has been able to measure clinical trials using their services as an undergird, against published industry performance sta- tistics – the story of the benefits of this new way of working with study patients is told by Validcare statistics mea- sured against in – dustry standards, and the results are clear,” commented Tylosky.
“For too long, sponsors have tolerated study delays and outright failures from their CRO partners. Running clinical trials is a pro- cess, with controllable variables and measurable outcomes. But traditional approaches fail to measure CROs on the processes that matter; resulting in delays, cost over runs and cancellations. This will never be the case with Farmacon Global’s clients as we work with the proven capacity and results offered in our new partnership with Validcare,” said Tylosky.
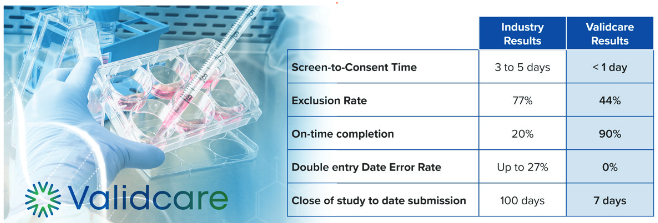
“With these tools Farmacon Global will achieve quick evaluation of ROI for activities intended to speed up trials like increasing patient enrollment, easier communication with principal inves – tigators and on-going participation. These proven technical services are especially critical for clinical trials in Latin America where Farmacon Global is uniquely positioned for delivering on-time and on-budget studies. Our focus has always been on the voice of the patients enrolled in a study and this strengthens it,” concluded Tylosky.
About Validcare: Founded by ex- perts with more than 20 years of industry experi- ence, Validcare offers the lead- ing solution for best practices in streamlining research for reg- ulatory compliant, life-improving products. Validcare brings a decade of experience powering clinical trials and value-based care models spanning pharma, med device and supplement market segments.

About Farmacon Global: Farmacon Global is a strategic CEO of medical consultants, pro- viding solutions to accelerate clinical trials in emerging markets – with a specialty in Latin America and emerging markets with in- place medical associates and KOL alliances. Specialties include rare disease, cancer, immunology and infectious disease.

